
SACCHAROMYCES CEREVISIAE CNCM I-3856 QUATREFLORATM : Patented Yeast Strain with Clinically Proven Efficacy in Protecting Against Vaginal Infections and Recurrences
Saccharomyces cerevisiae, also known as 'brewer's yeast', has been used since ancient times for its fermentation properties in the processes of making bread and beer. In addition to this yeast's interest in food, the strain Saccharomyces cerevisiae CNCM I-3856 has shown numerous benefits in combating fungal (Candida albicans) and bacterial (Gardnerella vaginalis) pathogens, both responsible for vaginal infections that impact women's intimate health and quality of life1,2.
Vaginal infections affect 75% of women during their lifetime. Bacterial vaginosis is the leading cause of vaginal infections with a prevalence of 40 to 50%, followed by vulvovaginal candidiasis (VVC), which represents 20 to 25% of cases. Additionally, half of the women experience a recurrence after a VVC, and 5 to 10% are affected by recurrent VVCs, with more than 4 episodes per year3.
QuatrefloraTM is a patented and ultra-stable strain of Saccharomyces cerevisiae CNCM I-3856 with clinically proven efficacy to help fight vaginal infections and prevent recurrences.
Several in vivo studies have highlighted the ability of Saccharomyces cerevisiae CNCM I-3856 QuatrefloraTM to :
- Form a protective barrier against pathogens at the vaginal epithelium1,4 ;
- Inhibit virulence factors of C. albicans and G. vaginalis1,4,5 ;
- Improve pathogen clearance by co-aggregation with C. albicans and by inhibiting the adhesion of G. vaginalis to vaginal and uterine cells1,4;
- Reduce inflammation and cellular damage caused by pathogens5.
A clinical study was conducted with 57 women divided into three groups of 19. The first group received a placebo, while the other two groups received 500 mg (2.5x109 CFU* of S. cerevisiae CNCM I-3856) and 1000 mg (5x109 CFU*) of QuatrefloraTM orally for 4 weeks.
Regardless of the dose, supplementation with QuatrefloraTM demonstrated the migration of Saccharomyces cerevisiae CNCM I-3856 to the vaginal level, thus highlighting its action at the site of infection. This microorganism was not found in women from the placebo group6.
A second clinical study was conducted over 56 days with 22 women presenting vaginal candidiasis. Between the 1st and 7th day of the study, all women were treated with an antifungal (Econazole ovule and cream). In parallel, 13 women received 500 mg of QuatrefloraTM (2.5x109 CFU*) orally, while the other 9 received a placebo. The supplementation with QuatrefloraTM or placebo continued after the end of the antifungal treatment, from the 8th day until the end of the study7.
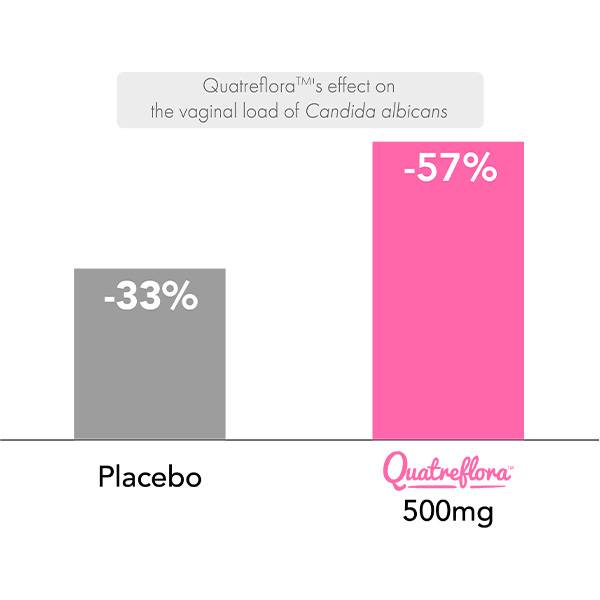
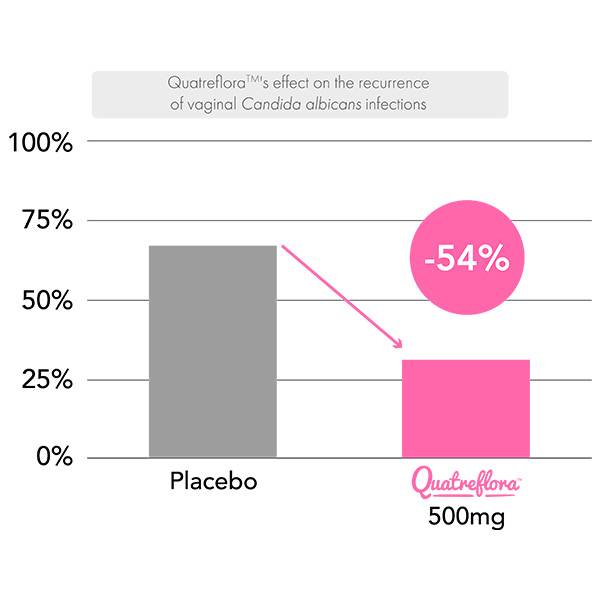
Supplementation with Saccharomyces cerevisiae CNCM I-3856 QuatrefloraTM for 2 months demonstrated a 57% reduction in the vaginal load of the pathogen Candida albicans compared to 33% for the placebo, accompanied by a 54% reduction in recurrences, thus confirming the efficacy of QuatrefloraTM to accelerate the clearance of C. albicans and highlighting its interest in combination with treatments for vaginal candidiasis.
*CFU : Colony Forming Unit

Bibliography
1. Wilson D. A tale of two yeasts: Saccharomyces cerevisiae as a therapeutic against candidiasis. Virulence. (2017) ;8(1):15-17.
2. Sabbatini S et al. Saccharomyces cerevisiae–based probiotic as novel anti-microbial agent for therapy of bacterial vaginosis, Virulence, (2018) 9:1, 954-966.
3. Sobel JD. Vulvovaginal candidosis. Lancet. 2007; 369: 1961-1971.
4. Pericolini E, Therapeutic activity of a Saccharomyces cerevisiae-based probiotic and inactivated whole yeast on vaginal candidiasis, Virulence, (2017) 8:1, 74-90.
5. Gabrielli E, et al. Saccharomyces cerevisiae-based probiotic as novel anti-fungal and anti-inflammatory agent for therapy of vaginal candidiasis. Benef Microbes. 2018;9(2):219-230.
6. Decherf A, Recovery of Saccharomyces cerevisiae CNCM I-3856 in Vaginal Samples of Healthy Women after Oral Administration. Nutrients. (2020);12(8):2211.
7. Cayzeele-Decherf A, Saccharomyces cerevisiae CNCM I-3856 as a Natural Breakthrough for Vaginal Health: A Clinical Study. Med J Obstet Gynecol, (2017);5(4): 1112.











Recommandé par mon médecin, je l'utilise régulièrement, avec de tvoir plus
Avis du 28/09/2024, suite à une expérience du 11/09/2024 par Silvia S